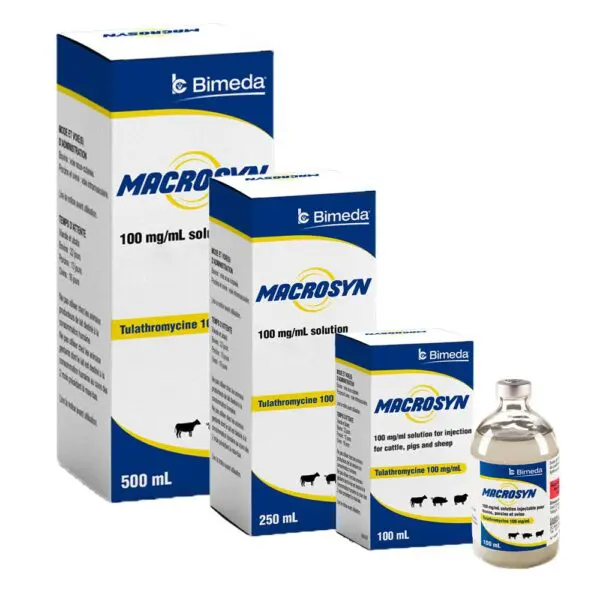
Description
Macrosyn injection is a ready-to-use injectable solution containing 100 mg of tulathromycin/mL, a broad spectrum, fast-acting antibiotic for use in cattle and swine.
Key benefits include:
-
Rapid absorption and distribution into body tissues.
-
Single-dose treatment and control for less animal handling.
-
Low-volume dosage means one bottle covers more animals.
-
Highly syringeable for easy use in any weather.
-
Short meat withdrawal.
Indications
Beef and Non-Lactating Dairy Cattle Treatment and control of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis; Treatment of infectious bovine keratoconjunctivitis (IBK) and bovine foot rot (interdigital necrobacillosis). Suckling Calves, Dairy Calves, and Veal Calves Treatment of BRD associated with M. haemolytica, P. multocida, H. somni, and M. bovis. Swine For treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica, Haemophilus parasuis, and Mycoplasma hyopneumoniae; and for the control of SRD associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, and Mycoplasma hyopneumoniae in groups of pigs where SRD has been diagnosed.
Specification
Package size |
100 , 250 , 500 Milliliter |
Species |
Beef Cattle, Dairy Cattle, Swine |
Active Ingredient |
|
Used for |
bovine respiratory disease (BRD), foot rot, pinkeye, swine respiratory disease (SRD) |
Used during |
Feedlot Arrival, Pre Conditioning, Weaning |
Route of administration |
Injectable |
Instruction
Cattle
Inject subcutaneously as a single dose in the neck at a dosage of 2.5 mg/kg (1.1 mL/100 lb) body weight (BW). Do not inject more than 10 mL per injection site.
Swine
Inject intramuscularly as a single dose in the neck at a dosage of 2.5 mg/kg (0.25 mL/22 lb) BW. Do not inject more than 2.5 mL per injection site.
Residue Warnings
Cattle
Cattle intended for human consumption must not be slaughtered within 18 days from the last treatment. This drug is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
Swine
Swine intended for human consumption must not be slaughtered within 5 days from the last treatment.

Reviews
There are no reviews yet.