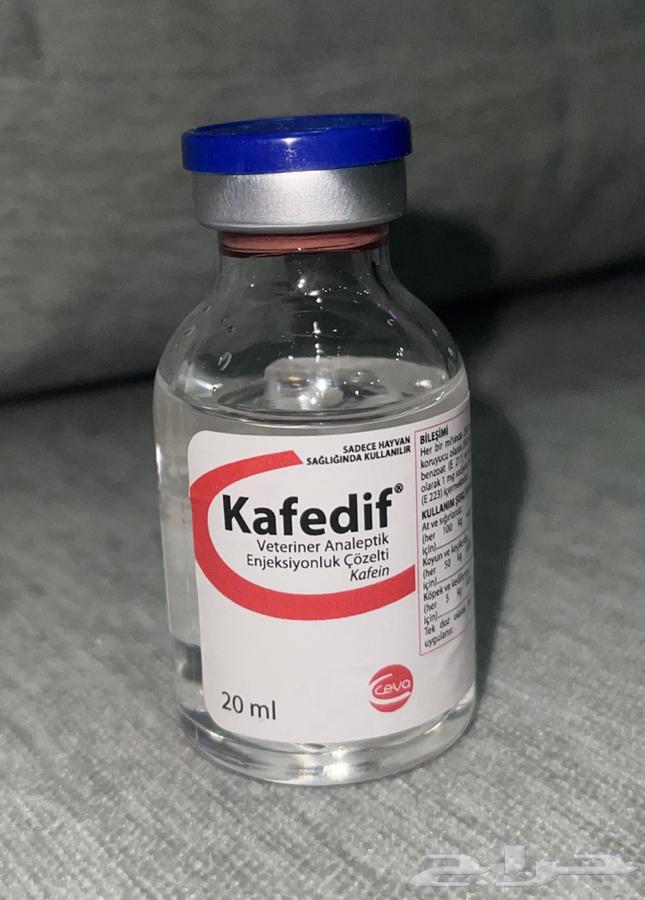
Description
Kafedif 20ml
Kafedif injection is a colorless injectable stimulant used in all cases where the central nervous system and respiratory system need to be stimulated.
In 1 ml; Contains caffeine 250 mg, sodium benzoate 350 mg. Ralgro (zeranol implants)
Kafedif Composition
It is a transparent, colorless, odorless, clear solution for injection. Each 1 ml contains;
Contains Caffeine: 250 mg
Sodium benzoate: 350 mg.
PHARMACOLOGICAL PROPERTIES
The active ingredient of Kafedif is caffeine, a xanthine-derived alkaloid. Kafedif is a very effective CNS stimulant. Although the heartbeat slows down initially due to the stimulation of the central nervous system, after a short time it regularly accelerates the heartbeat by stimulating the heart muscle. Thus, the power of the heart increases, the pulse accelerates, blood pressure rises and the heart is better nourished as the coronary vessels expand. It increases contraction and effort power by stimulating striated muscles (Doping).
Kafedif shows its effect by inhibiting the activity of phosphodiesterase, which breaks down sAMP, and extending the half-life of this nucleotide in cells.
Kafedif is well absorbed from the application site and distributed throughout the body. 80-85% is excreted in urine within 48 hours. Soma-Max-10
SPECIAL CLINICAL AND INFORMATION AND SPECIAL WARNINGS FOR TARGET SPECIES
Due to the doping effect on racehorses, the application should be stopped 10 days before the race.
DRUG INTERACTIONS
It does not have any interactions with other medications. Can be used together.
KAFEDIF SYMPTOMS, PRECAUTIONS AND ANTIDOTE IN OVERDOSE
High doses may cause restlessness, insomnia, excitement, and intermittent and continuous convulsions. Respiration accelerates, vomiting and expulsion are observed. These effects can be blocked by barbiturates.
UNDESIRABLE/SIDE EFFECTS
It dilates peripheral vessels, so it may cause a drop in blood pressure. It causes an increase in oxygen consumption, increase in body temperature, sweating, tremor and increase in muscle tone in animals.
WARNINGS ABOUT DRUG RESIDUES IN FOOD
Drug residue purification period (ikas): The residue purification period for meat and milk in cattle, sheep and goats is “0” days. There is no harm in sending cattle, sheep and goats to slaughter during the treatment and after the last drug application. Cow, sheep and goat milk obtained during and after drug use can be offered for human consumption.
CONTRAINDICATIONS
Kafedif treatment is a drug with a wide range of safety. However, in high doses, it may cause restlessness, insomnia, excitement, and intermittent and continuous convulsions. These effects can be prevented with barbiturates. Parenteral administration to cats and dogs in high doses produces lethal effects.
USE DURING PREGNANCY
There is no harm in using it in pregnant animals.
KAFEDIF 20ML GENERAL WARNINGS
Consult your veterinarian before use and if any unexpected effects occur. Keep out of reach of children and away from foodstuffs. Do not purchase or use products whose shelf life has expired or whose packaging is damaged. Vigantol ADE Fuerte 100ml
STORAGE CONDITIONS AND SHELF LIFE
Store in its own packaging, in a cool and dry place, at room temperature (15-250C). Shelf life is 5 years from the date of manufacture.
COMMERCIAL PRESENTATION
It is marketed in 10 ml and 20 ml transparent glass vials in a cardboard box.



Reviews
There are no reviews yet.