Description
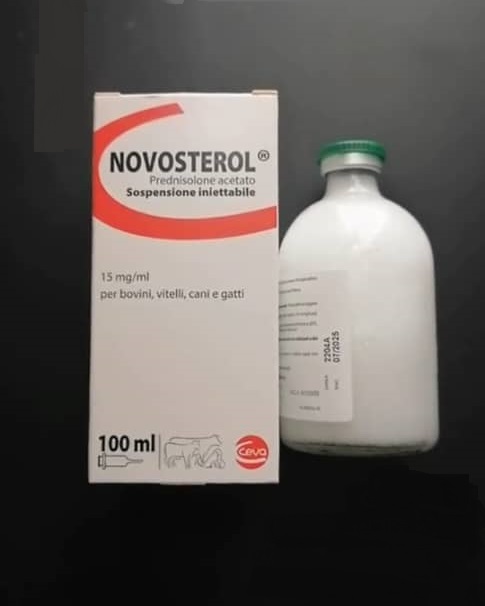
Novosterol 100ml
Novosterol 15mg/ml injectable solution for the treatment of cattles, dogs and cats in athological conditions related to pregnancy and childbirth (pregnancy toxicosis, acetonemia or ketosis); acute conditions affecting the musculoskeletal system (articular rheumatism, bursitis, tendonitis, tenosynovitis); skin disorders (allergic dermatitis, eczema, urticaria, general itching); states of shock and asthmatic bronchitis.
Novosterol is a veterinary medicine based on the active ingredient Prednisolone Acetate , belonging to the category of Corticosteroids and specifically Glucocorticoids Phenylbutazone 20% Injection, 100 ml
ACTIVE INGREDIENTS
Each ml contains, active substance: prednisolone 13.4 mg (equivalent to prednisolone acetate 15 mg).
EXCIPIENTS
Polyvinylpyrrolidone, polyethylene glycol 4000, sodium chloride, benzalkonium chloride 0.10 mg, sodium hydroxide, water for injections.
NOVOSTEROL 100ML DIRECTIONS
Cattle, dogs, cats: pathological conditions related to pregnancy and childbirth (pregnancy toxicosis, acetonemia, or ketosis); acute conditions affecting the musculoskeletal system (articular rheumatism, bursitis, tendonitis, tenosynovitis); skin disorders (allergic dermatitis, eczema, urticaria, itching in general); states of shock and asthmatic bronchitis.
Calves: acute conditions affecting the musculoskeletal system (articular rheumatism, bursitis, tendonitis, tenosynovitis); skin disorders (allergic dermatitis, eczema, urticaria, itching in general); states of shock and asthmatic bronchitis.
CONTRAINDICATIONS/SIDE EFFECTS
Do not use: in case of heart disease, kidney disease, degenerative eye disease, diabetes mellitus; in case of viral, parasitic and fungal diseases; in advanced pregnancy (may cause abortion); in osteoporosis.
Do not use in cases of hypersensitivity to the active substance or to one of the excipients. Bleeder Breather Jug 100ml
SAFETY IN REF. SPECIES
Data not available. Do not exceed the indicated doses.
USE/ROUTE OF ADMINISTRATION
Intramuscular use.
DOSAGE OF NOVOSTEROL INJECTION
Intramuscular use: cattle 90-210 mg prednisolone acetate/head (equivalent to 6-14 ml);
calves 60-90 mg prednisolone acetate/head (equivalent to 4-6 ml);
dogs and cats 15-30 mg prednisolone acetate/head (equivalent to 1-2 ml).
The rubber stopper of the bottle can be safely punctured up to 20 times. Shake before use.
CONSERVATION
Store below 25 degrees C. Store in the container to protect from light.
Shelf life of the veterinary medicinal product as packaged for sale: 36 months.
Shelf life after first opening the primary packaging: 28 days.
NOVOSTEROL WARNINGS
Special precautions for safe use in the target species: Corticosteroid therapy temporarily suppresses the manifestations of inflammation and itching, but does not act on the causes of the disease.
Frequent repetition of administrations for the treatment of relapses is not without risks. In case of concomitant bacterial or viral infection, the animal must first be treated for these pathologies.
The veterinary medicinal product must be used with caution under direct veterinary supervision in case of muscle hypotrophy, chronic debilitating diseases and slowly healing cicatricial processes.
The administration of cortisone should be avoided in late pregnancy as it may cause premature birth or abortion. After prolonged corticosteroid therapy, gradually discontinue administration by reducing the dosage to avoid an adrenal insufficiency crisis.
The immunosuppressive effect may decrease resistance to infections or exacerbate existing ones. In the presence of viral infections, corticosteroids may accelerate the progression of the disease Dexaphenylarthrite 100ml



Reviews
There are no reviews yet.